|
A New Model for Atomic
Structure
by Cuong
Nguyen
Abstract
A
better model of atomic structure is presented, and hopefully to resolve many
questions that have baffled scientists for decades. It explains how primordial particles were created in the first
place, and what had really happened before the Big Bang. Furthermore, the model can be mathematically
proved, and verified from other historical experiments conducted by our
renowned scientists in the past.
Introduction
The
nuclear physics was primitively introduced by Thompson's experiment with the
cathode rays more than a century ago. Its powerful application only began six
decades ago when the first atomic bomb was designed and tested in New Mexico.
Since then, nuclear engineering has resulted in hundred useful applications
including nuclear energy, medical treatment and even on the key component for a
life-saving, simple device likes smoke detector in houses around the
world. But, while so many research
works with impressive achievements being realized by our best scientists, one
may still wonder whether we truly understand the atomic structure of a simple
element, such as hydrogen?
The
true answer might be a humble "No". The
best minds in the science community are still unsure how atoms were formed in
the first place. As of present time,
most of our scientists believe that a nuclei of an atom includes at least
several subatomic particles: Quarks, Gluon and Muon, etc., which help bonding
the primary particles together, proton and neutron. On the other side, however, there are still quite a few skeptical
people including me, who's not satisfied with the current theory of atomic
structure. We believe that those
subatomic particles could not be found or identified without smashing atoms in
a giant accelerators, artificially.
It
is true that the Big Bang event actually produced massive collisions and
extreme heat within the particles, so that protons and neutrons could be
smashed and broken up into smaller fragments which still carried small positive
or negative charge. As a result, they
were attracted to each other and merged back to form the original
particles. But the reunion should have
not been mistaken for the naturally made, subatomic particles.
As
of present time, we still do not know exactly how protons and neutrons are
arranged in a nuclei, and how electrons are orbiting around its nuclei. The best theory of quantum mechanics may
explain reluctantly how electrons are circling outside of a nuclei. After all, we have been fortunate that most
nuclear applications have never required scientists to calculate or use any of
the subatomic particles, except the three most basic particles: Electron,
Neutron and Proton.
According
to Darwin's theory of evolution, any object in this universe should be evolved
gradually into a more complex form. If
Darwin is right, his theory also means that any matter, from the beginning of
our universe, should be formed by a very simple way, if not the simplest one.
The theory presented in this paper will confirm the theory and hopefully would
resolve other remaining issues as well.
Before the Big Bang.
The
Big Bang theory was initially mentioned by Hubble in the decade of 30s,
suggested later by Gamow and actually confirmed by the discovery of cosmic
radiation background in 1964. There have been findings that included recent
photos taken by space stations, showing similar explosions that have been
occurred somewhere in the deep space beyond our milky galaxy. The nuclear
physicists also agree that the Big Bang event was similar to a thermonuclear
explosion created by means of nuclear fusion.
As a result, we have no doubt about the Big Bang theory because the hard
evidences are very convinced. But the
debates would not stop here. It seems
to raise more questions and it goes furthermore to challenge our intellectual
curiosity. The most frequently-asked
questions are: If indeed, there was a Big Bang to start the universe, then what
had really happened before? More
importantly, what was the fuel to power the Big Bang? And how was it made?
The
theory of atomic structure presented in this article may not be conceptually
formed without understanding its common logic.
As mentioned previously, any matter that had appeared first, either
Proton, Neutron or something else, had to be formed by the most simple way, if
not the simplest one in the history of our universe. So, if any existing object in our universe had been gradually
evolved to a more complex form now, then, looking back its original in the
first place, it had to be created by a very simple form.
There
had to be a period called "Incubation Period" which could take up to dozen of
billion years to prepare the fuel, long before the Big Bang event or the first
cosmic explosion. No matter who made it, or how the Big Bang was begun, the
first Big Bang explosion had to have something that physically called "fuel" to
start with.
It
was suggested and later accepted by many scientists that the Big Bang was
created by a chain explosion, similar to the nuclear fusion of the hydrogen
elements. In deed, the "Incubation
Period" was to produce that kind of fuel.
The remaining critical question:
How was the Universe begun? The
answer is the following theory of the "Small Crack" (One may think of the "Big
Silence", instead)
The Small Crack
From
the very beginning, our universe was in a singularity state, or philosophically
nothingness, i.e. all was absolutely the same. The size of the universe at that
time could be speculated as small as a point on this page, or as big as our
existing one. Then appeared a very
"Small Crack" (Please don't ask who did it!).
After the first "Small Crack" had been occurred, the surrounding area
including the upper and lower portion, were reacted to the first crack as if
they had just lost something, rushed back to retrieve its lost which was
actually a little "void" created by the crack. Consequently, both upper and
lower bodies were pulled away and left their main body, causing a chain
reaction behind them, creating more cracks and voids.
After
all, the primitive universe had just begun a process of "incubation period",
when more cracks and voids were created.
For illustration, we may recall seeing a broken windshield of cars after
they were involved in a massive collision.
The first act of retrieving the lost ones between the two bodies and the
little voids will be later called the attractive force between positive and
negative elements. We may also assume,
by coincidence, the size of a void was as small as an electron, and the two
little separated bodies were as big as a proton or a neutron.
Now,
let's just imagine there is a big dancing party for single people. It would be no problem if every participant
could have his or her interesting partner to dance with! But, what would happen if there are two
people who may be interested in one partner? Well, there are two best scenarios
that would likely happen: Either one
has to give up, or one of them would have to share or take turn to dance after
another.
That
is the case exactly happened from the dawn of our universe. Again, let's think
back to the moment after the first cracks had happened. Due to their almost equal strength, there
was a tie and then a little fight between the upper and lower bodies to
retrieve the void. For our convenience, let's name "X" for the "Positive"
(upper or lower bodies) and "Y" for the "Negative" (void). The physical
characteristics of those first two elements in the universe were primarily
established. As of that time, X and Y
were not really Proton and Electron yet until after the Big Bang. They were
just in primal state with a tendency to become either Positive or Negative.
Both X had tried to take over Y, and because of their equally attractive force,
no one could clearly win the contest.
As a result, Y was being pulled back and forth between the upper and
lower X.
The
original speed of Y when running back and forth between two X's was as slow as
anyone could image. From the beginning,
it would take years or even longer to have Y complete a full cycle of "back and
forth"! By then, the primitive atomic
structure had included only a little Y which had been moving back and
forth between the two big Xs.
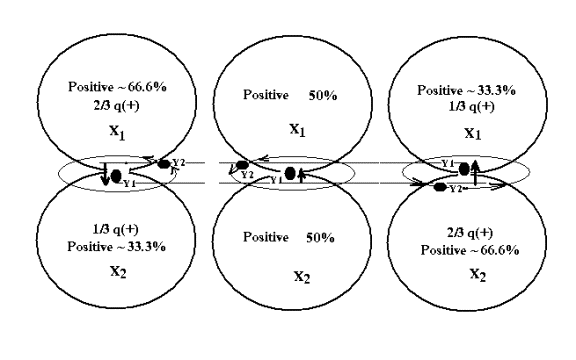
Figure
1: A complete cycle of Primal Deuterium
(or Hydrogen) was shown in 3 stages.
The
ratio of holding Y between two Xs was very small from beginning and gradually
increased to an ultimate ratio that was
allowed for a stable structure. The
ultimate ratio could never reach up to more than 66.6-33.3 ratio (The ratio is
an assumption and will be verified mathematically later in this article). It should also be noted that a nuclei would
never have a pure state (100%) of a single proton or neutron, unless its atomic
structure collapses, then either proton
or neutron would be completely thrown out of its nuclei.
Primal Deuterium.
Almost
at the same time, another free and available void Y(2) from surrounding area
was also attracted and moved toward the most positive X (say X(1)),whichever
was available to most attract the second Y (figure 1). While Y(2) was moving
toward X(1), Y(1) was also pulled back into the same X(1), which was also
changing to less positive. At that moment, Y(2) was attracted to other X(2) and
changed its course moving toward X(2).
Alternatively, Y(2) direction was changed back and forth, and finally
circling around the alternating Y(1).
For illustration of the event, the whole episode may be liken to a dog
chasing a ball that being thrown back and forth between two players. The
phenomenon was later described as the electromagnetic force in an atom right
after the Big Bang.
After
trillion by trillion of cycles or more, due to symmetrically and equally
attractive force from both X(1) and X(2), Y(2) movement was steady and orbited
around in the Y(1)-Y(2) plane (figure 1).
Its circular orbit was eventually in a plane perpendicular to the X1-X2
axis. Furthermore, because more energy
was being slowly built up after trillion by trillion of orbits Y(2) had made,
the speed of Y(2) was increased and so did the Y(1). The first Primal Deuterium(P-Deuterium) was finally formed, few billion years after the "Small Crack"
had happened.
Primal Helium.
Few
more billion years passed after the first P-Deuterium had been made, the
universe was like a "bowl of soup" with primal deuterium (P-Deuterium)
elements. Because the interactive
force between any two P-Deuterium and the energy accumulated, the more
P-Deuterium were made, the higher speed for Y(1) and Y(2) was obtained in that
"bowl of soup". Also, any two nearby P-Deuterium were being slowly repelled
each other. The intensity of the
repelling force gradually increased, and consequently, creating more space so
that some P-Deuterium could freely move around, turned over upside down and
become "negative" P-Deuterium (i.e. 180 degree rotation of the existing
P-Deuterium. It is the same P-Deuterium but having opposite signs, negative to
positive, or vice versa). And then
appeared two types of negative and positive P-Deuterium.
After
negative P-Deuterium had appeared, it was attracted and merged with a positive
P-Deuterium. The two combined P-Deuterium yielded a Primal Helium (P-Helium), which is the most basic element to create
all other elements in the universe (Fig. 2).
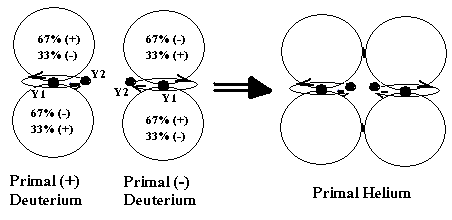
Figure
2. P-Helium was formed by Positive and
Negative P-Deuterium.
When P-Helium was formed, the two P-Electrons
were orbiting in opposite direction, clockwise and counter-clockwise (Fig. 2).
Later when the two orbiting P-Electrons reached near the speed of light, their
orbits became larger and moved closer, and they were influenced physically to
each other. The encounter of two
P-Electrons made them kicking each other and spinning in the opposite direction
with its orbit, i.e. clockwise for negative spin (-) and counter-clockwise for
positive spin (+).
Primal Tritium.
Our
primitive universe was then looked like a cross section of a tree trunk. At the center was full of P-Helium and
P-Deuterium. The next layer contained
most of P-Deuterium mixing with less P-Neutron which were in a process of
making P-Helium. The outermost layers included P-Proton, P-Electron and
P-Neutron making P-Deuterium, etc. The whole manufacturing process was
continued in a chain reaction spreading from the center to the outer
perimeter. The more P-Helium were
created at the center, the more P-Deuterium were made. In general, the energy for each primal
element was proportionally increased with newly created ones in the primitive
universe. The speed of P-Electrons
which were orbiting outside, or moving back and forth inside a nuclei, also
increased, accordingly.
When
a vast pool of P-Helium being built up, the energy created by interactive
forces between the P-Helium elements were also increased, and so was the speed
of the captured P-Electron inside the nucleus axis. Based on what we have learned so far, it could take few more
billion years for the speed of the captured P-Electron which located inside a
P-Helium nuclei to reach critical level, probably two-third the speed of
Light. At this critical speed, movement
of some P-Helium could be disrupted, the inside P-Electron was lost and
captured totally by either one P-Proton, turning it into a permanent P-Neutron.
Consequently,
the remaining P-Proton was repelled by other nearby P-Proton and kicked out of
the P-Helium element, freeing other P-Electron too (Figure 3). The first Primal Tritium (P-Tritium)was
made. Its trillion by trillion of P-Tritium came up later by the same process,
and that was the last and final step to make fuel for the first Big Bang event.
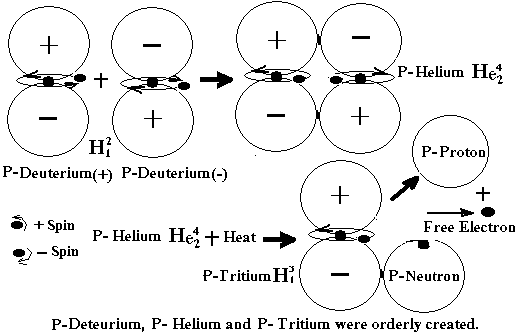
Figure
3. The process of making P-Tritium from P-Helium before the first Big Bang.
The Big Bang
Finally,
after dozen billions years later since the "Small Crack" had occurred, our
primitive universe was like a giant layered sphere with most P-Tritium at the
center mixing with outer layers of P-Helium, P-Deuterium, P-Neutron, P-Proton
and P-Electron. In fact, it had all
ingredients for a fusion explosion waiting to be triggered when the time came. The D-time possibly came on after energy had
been gradually built up to the most critical level for a nuclear fusion between
P-Helium and P-Tritium.
Then
a "Big Boom" and a chain cosmic explosions we may call the Big Bang event had
finally happened and lasted forever. It
is now confirmed by some photos showing the cosmic explosions similar to the
Big Bang events are continuously happening somewhere near the outer edge of our
universe. We really don't know how long
it took from the "Small Crack" to the first "Big Bang" event. But for a good estimate, the whole episode
could have taken up at least as long as the time passed from the "Big Bang" to
our present time, i.e. somewhere from 15 to 20 billion years.
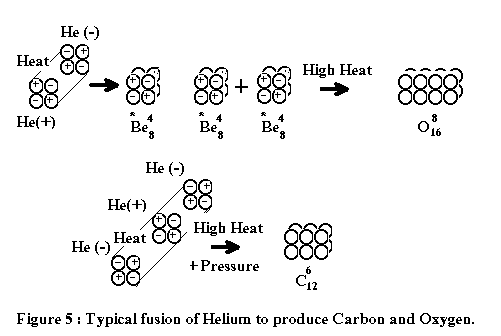
After
the "Big Bang" event or first cosmic explosions had occurred, the first basic
elements like Carbon, Oxygen, or heavy metals were formed under extreme heat.
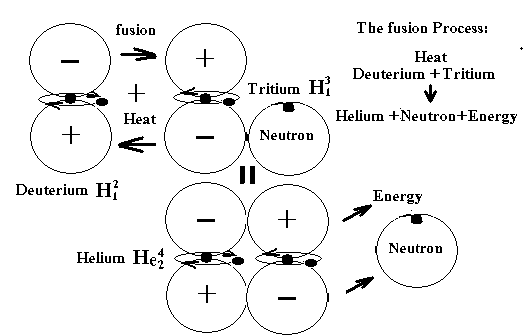
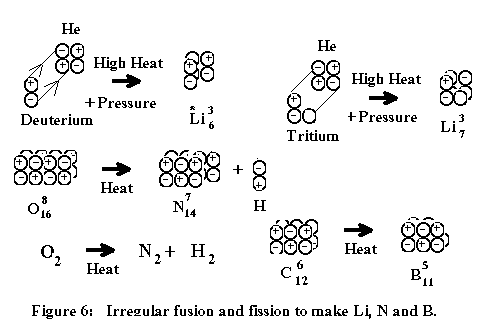
Figure
4. The fusion process happened during the Big Bang event.
Atomic structure of the first elements.
The
"Small Crack" theory would not be substantiated or proven if it can't explain
how the fundamental elements such as Carbon, Oxygen and Nitrogen etc. were
formed or created in the first place.
In this topic, a theory of the atomic structures will be explored in
depth.
Few
seconds after the Big Bang, the immerse heat and collision caused more fusion between
the first basic elements to create other light elements. The most basic form for this important task
was Helium, also the first by-product of fusion as shown in Figure 4. By then, within a vast pool of Deuterium,
Positive Helium and Negative Helium were combined to produce Barium Isotope (Z = 4, A
= 8), Carbon, Oxygen, Nitrogen, and so on...
Now,
let us review mathematically the atomic structure of a very basic element of
our universe after the big bang: Hydrogen atom. In the next exhibition, at first we establish a formula for
calculating the speed of electron orbiting around OO' axis, and then calculate
the speed when it reaches to maximum distance which is equal to a proton
radius. As shown below, the attractive
force between two alternate Proton and Neutron and the electron orbiting
outside is: F = k . q . q . Cos q / (OE **2)
The
above electric force must be equal to the centripetal force created by the
orbiting Electron. ( Because the electric force is always much stronger than
the gravitational force, we can ignore the gravitational force in the
calculation.)
All
other calculations are shown in the figures below:
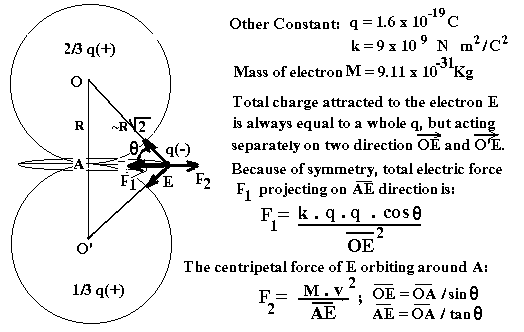
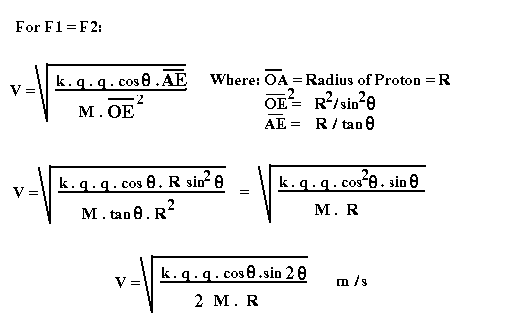
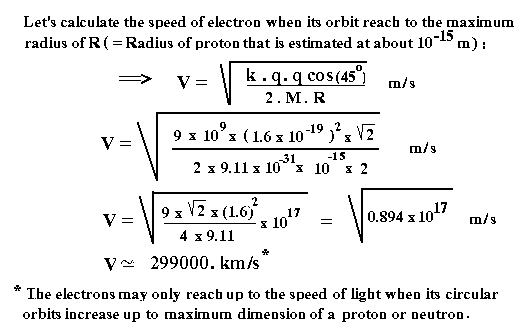
Based
on the theory presented, there is no hydrogen atom with only one proton and one
electron. The only existing form of Hydrogen is Deuterium (also called heavy
water). As described previously, the
atomic structure of Deuterium includes two similar particles of a temporary
Proton and Neutron that are connected by a moving electron inside and another
electron orbiting outside between the two particles (figures 3&4).
The
following figures illustrate how the first elements were formed and created
after the Big Bang event:
Rutherford's experiment
The
Rutherford's famous experiment with alpha particles and very thin foils of gold
can be explained as follows:
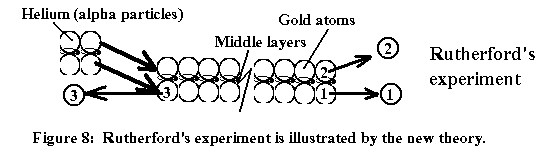
There
are three cases described in the experiment as shown on the above figure 8:
1: The alpha particles actually never
penetrated through the gold foil, but protons and helium at the other side were
released, instead. When bombarding the
gold atoms at the target side, electrons inside were energized and heated up to
reach the speed of light. By chain
reaction, the electrons finally escaped its atoms at the other side of the gold
foil. The lost of electrons made gold
atoms unstable. As a result, most particles or helium elements at the
other side were repelled and ejected out following direction 1-1.
2: A few electrons had reached speed of light
and escaped late. Therefore, the
remaining particles were not only repelled late but also deflected (by particle
1 ) to the direction 2-2.
3: Very few others at the target side were
simply repelled and kicked back out at 3-3, because its atoms had lost
electrons and became unstable after being bombarded by alpha particles.
There
were two other experiments conducted by Rutherford (1919) and Chadwick(1932):
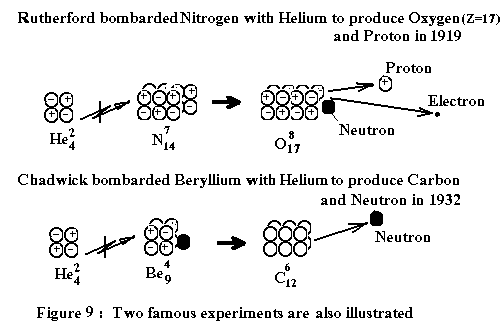
Recently,
there also were a couple of interesting news coming out from the universities
and research institutes. The
researches, by coincidence, illustrate the theory presented. The first was from UCLA where the physicists
claimed to have created nuclear fusion at room temperature. A portable device was built to make two
Deuterium nucleus collide with each other and create a fusion process. The result for that fusion was to produce
Helium, Energy and Neutrons (as illustrated in the figures 3&4). The only problem for the device is that it
required more energy to operate than the fusion can produce.
The
second was published in the Japanese Journal of Applied Physics by Iwamura et
al. at Mitsubishi Heavy Industries Advanced Technologies Center. The research reports a chemical experiment
called transmutation phenomenon where Cesium (A=55; Z=133) was
gradually disappeared and replaced by Praseodymium (A=59; Z=141) without any contamination or interference from other
sources. The same test were
successfully repeated with other elements like Strontium (A=38; Z=88) to Molybdenum (A=42; Z=96). The
results somehow confirm that there were either two Helium (A=2; Z=4) or a Beryllium isotope B+(A=4; Z=8) added
easily to the first elements and changed them to totally different species.
Bonding between elements.
The
important topic for this section is to explain how the elements would bond with
each other to create chemical compounds.
As illustrated in the above figure 9, two different elements are bonded
together by the same "attraction" force at either one of both ends. However, the big difference is that the axis
of alignment for two different elements are perpendicular to each other (i.e.
the Y and Z-axis are rotated 90¡ã along X-axis for the second element).
We
may wonder why the bonding of two elements could not happen on the other Y-axis
or Z-axis? The answer is: Yes, it could, but that kind of bonding could have
big problem for creating mass of its molecules. Consequently, any compound created by an abnormal bonding could
not become a common substance that we can easily see in vast quantity.
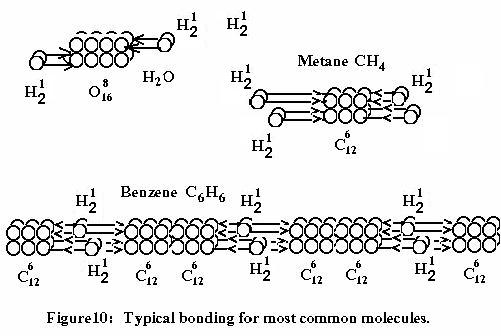
Another
typical question: If the bonding happens as illustrated in the above figure 9,
and if we have CH 4, why can't we have the substances like OH 4 or SH 4, etc.?
The
answer: Because the elements O(Z =16) and S (Z = 32) have higher Z and stronger magnetic field that could
influence the speed of electrons and destabilize H atoms at both ends.
Otherwise, Carbon (Z = 12) may
have less magnetic influence, and most of all, CH4 is always in the state of
gas which is the weakest bonding of a chemical compound.
Cuong Nguyen
|

